Fmri in Psychiatry an Advanced Literature Review Solimine Nguyen
Introduction
Schizophrenia (SCZ) is a major psychiatric disorder characterized by positive and negative symptoms, associated with cognitive harm, leading to a worse consequence and a high bear on on global functioning (ane). The lifetime prevalence is 0.40% (ii), and it has been estimated that approximately 1 in 200 individuals will be diagnosed with SCZ at some indicate during their lifetime (3). Fifty-fifty if the diagnosis of schizophrenia is fabricated by observation of the clinical features of the disorder according to the Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5) (4) or on the ICD (v) criteria, evidences on specific biomarkers that can predict or notice the illness accurately at an early stage are still deficient. (6). Information technology is clear that, considering the biological complication, the endeavor to improve insights into the disease processes is difficult: encephalon neuroanatomy is intrinsically complex and heterogeneous (7). Not-invasive measurements of encephalon function and structure, as neuroimaging, are useful and powerful tools for studying discriminatory biomarkers (8, 9) in patients with mental disorders. In this regard, brain imaging studies take revealed that functional and structural encephalon connectivity in the default mode network (DMN), salience network (SN) and central executive network (CEN) are consistently altered in schizophrenia (x). To date, functional MRI (fMRI) and structural MRI (sMRI) represent the most used techniques to provide a multiple perspective on brain part, construction, and its connectivity. Big amounts of imaging data from magnetic resonance imaging (MRI) need to be analyzed by computerized methods that are able to process data and determine the probability of diseases with great precision (11). Ascension attending has been given to motorcar‐learning (ML) techniques (i.e. pattern recognition methods) applied to neuroimaging data (12) to identify phenotypes to be translated into clinical practice for early diagnosis (thirteen, 14). ML techniques applied to fMRI analyze highly circuitous data sets and assess the importance and interactions between variables, exploring brain functionality and making accurate predictions (xv, 16). Motorcar learning stems from the theory that computers can larn to perform specific tasks without being programmed to exercise so starting from specific input, thanks to the recognition of patterns in the information. Automobile learning uses algorithms that acquire from information iteratively. For case, information technology allows computers to notice data, fifty-fifty unknown, without being explicitly told where to look for it (17). Amidst them, the Support Vector Machine (SVM) represents ane of the ML techniques that has shown higher accuracy and precision especially in predicting clinical outcome and severity in schizophrenia patients (xiv). SVM is a supervised learning model with associated learning algorithms that analyzes information used for classification and regression analysis. This technique has yielded skilful results applied to fMRI in defining a fix of features and information from the diverse regions of the brain allowing to classify healthy controls and patients affected past SCZ with a potential great translational impact (11).
This review aimed to appraise the current state of the evidence about the use of SVM techniques in making diagnostic bigotry in SCZ patients from salubrious controls (HC) using as input neuroimaging data from fMRI, co-ordinate to PRISMA guidelines (18).
Materials and Methods
Search Strategy
Articles published until September 27th, 2019 in PubMed, Embase, MEDLINE, PsychINFO, and the Cochrane Library, without language and time limits, were searched by using the following keywords: (Deep Learning OR DL OR Large information OR Artificial Intelligence OR Car Learning OR Gaussian procedure OR Regularized logistic OR Linear discriminant analysis OR LDA OR Random wood OR Least Absolute selection shrinkage operator OR rubberband cyberspace OR LASSO OR RVM OR relevance vector machine OR pattern recognition OR Computational Intelligence OR Auto Intelligence OR support vector OR SVM OR Pattern classification OR Deep learning) AND Schizophrenia AND (fMRI OR magnetic resonance imaging OR MRI OR functional MRI OR functional-MRI OR functional magnetic resonance imaging). All the selected studies were individually reviewed by ii researchers. Reference lists from the included articles were screened for additional studies. The eligible publications accept been included and cited in this review.
Assessment of Written report Quality
In this systematic review we applied the Jadad rating organisation (19) to check the methodological quality of included studies. Jadad'southward procedure allows to qualify selected studies according to their transparency and reproducibility, with bang-up validity and reliability evidence, through the clarification of iii simple and easy items: randomization methods, the double-blinding process, and the patient's withdrawal and dropout reports. Scores range from 0 to five points. The cut-off for inclusion in this study was a Jadad score ≥iii.
Selection Criteria
We selected studies applying SVM every bit ML techniques with patients diagnosed with Schizophrenia according to the DSM-4, DSM-Iv TR, DSM-5 or ICD-ten criteria, chronic SCZ or at starting time episode of schizophrenia (FES) regardless of antipsychotic medications. We excluded studies without a control group and trials including patients afflicted by general medical conditions, neurological or psychiatric comorbidity, substance corruption or alcohol dependence, traumatic brain injuries with loss of consciousness, and unclear or unverified psychiatric diagnoses according to the DSM or ICD criteria.
Information Drove and Extraction
Two authors (RdF and EAC) independently screened all the titles and abstracts of the collected articles, and fully read the texts of papers that met the eligibility criteria. In cases of disagreement, a tertiary researcher (LS) supervised and made the final decision. Data from the extracted commodity included: publication twelvemonth, sample size, diagnoses, and all statistical data and features (i.e. accuracy, sensitivity, specificity, brain region or networks).
Results
Initially, 660 items were identified, of which 384 manufactures were eliminated considering they did not fulfill the inclusion criteria. The abstracts of the remaining 276 articles were reviewed. Overall, 226 out of 276 articles were excluded because they were non trials (i.e. editorials, messages to editors, reviews, meta-analyses, case reports or unlike interventions). Then, 28 manuscripts out of 50 papers were further excluded considering they did non fulfill the inclusion criteria (e.g. unclear or unverified psychiatric diagnoses, studies considering consequence, costs or therapy or not using MRI); the remaining 22 studies (Table ane) were included in this review (Figure one).
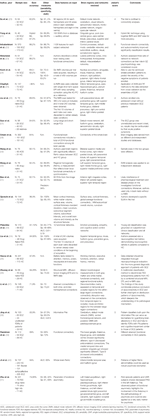
Table 1 Summary of included studies classifying schizophrenia using SVM.
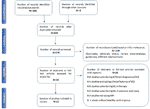
Figure 1 PRISMA flowchart of included studies.
Discussion
Included studies were very heterogeneous, and the samples vary in size and clinical characteristics (Tabular array 1). Several features from different brain regions were used as inputs for SVM and focused to investigate how the functioning of the model in accuracy, precision, sensitivity, and specificity could be affected by these variables. Studies in this review mostly used and evaluated frontal, temporal, and occipital brain regions. ML techniques were able to detect significantly altered activation patterns or brain connectivity differences in SCZ patients compared to HC. Moreover, this happened quickly, finer, and efficiently, greatly reducing the number of imitation negatives, as desirable for a good screening test (42, 43). SVM has accomplished skillful results in terms of accurateness and precision in identifying patients with SCZ. This technique tin can amend the clinical and research tasks due to the repetitiveness of the data. Computers larn from previous processing to produce results and make decisions that are reliable and replicable (17). SVM presents pros and cons. Specifically, an important advantage is that SVM is the about used and well-known machine learning tool, and fifty-fifty when other techniques are validated, they are compared with SVM. It achieves high accurateness level (e.g. 99%) and is the golden standard to develop new techniques. It can exist used for both classification and regression purposes; information technology allows information repeatability; it can be used in dissimilar fields of study, and it represents a smashing selection for hereafter studies. Nevertheless, it is expensive, and its interpretation is not uncomplicated as information technology requires an experienced and dedicated team (xiv, 44, 45).
Pläschke et al. used the resting-state Functional Connectivity (FC) to differentiate SCZ patients from matched HC, reaching a remarkable accuracy, equal to 68%. Interestingly, emotional scenes and face processing, empathic processing, and cognitive action control have proven to be the best networks to accurately discriminate patients from HC. Moreover, the age affects network integrity in a more global way so it could be used as a specific flag of functional dysregulation in particular networks afflicted in SCZ (33). The results of Bae's written report reported a decrease in the global and local network connectivity in SCZ patients compared with HC, especially in the superior right temporal region, in the inductive right cingulate cortex, and the inferior left parietal region with an accuracy of 92.1%, sensitivity of 92%, specificity of 92.1% and precision 94% (31). One of the largest studies on SCZ (200 patients vs 200 HC) reported a high diagnostic accuracy (84%) using data from several locations. Otherwise, significantly poorer accurateness was reached with the utilize of individual sites, showing a lower connectivity in SCZ patients (28). Su et al. recreated the whole brain functional connectivity in SCZ patients (23) vs HC (23) and related the exact spatial location of the activated brain areas to the emerging symptoms. With >80% accurateness authors found an increased FC in SCZ patients group (20). Information technology could probably be explained by an altered cerebral connectivity spread throughout the whole brain, with particular aberrations constitute in many of the master connections. Contradistinct connectivities in both intra- and inter-hemispherical connections were observed past Li et al. (37), especially in the right hemisphere more than than the left hemisphere (temporal, occipital, insula, and limbic regions). Similar data were confirmed in others studies focusing on altered connections (decreased in the basal ganglia, thalamus, lingual gyrus, and cerebellar vermis and increased in medial temporal lobe and posterior cingulate gyri) (39). Koch et al. reached 93% accuracy in identifying SCZ patients and were also able to predict the severity of the negative symptoms of patients based on ventricular striatal activation patterns (24). The results of these studies corroborate the idea of the occurrence of dysconnectivity in schizophrenic patients and deepen our cognition on the pathological mechanisms.
Functional network connectivity (FNC) to capture the internetwork connectivity design and autoconnectivity to capture the temporal connectivity of each brain network were proposed as features for SVM technique (22). The authors manage to reach particularly high accuracy values in guild to discriminate patients with SCZ from HC cheers to the integration of these features (autoconnectivity + FNC). Indeed, the terminal diagnostic and classification accuracy settles in 88.21% (83.7% for FNC and lxxx.ii% for autoconnectivity alone), with a sensitivity of 86.7% (81.iv% for FNC and 78.1% for autoconnectivity lonely) and a specificity of 89.5% (85.9% for FNC and 82.2% for autoconnectivity solitary). In one of the first studies, the authors were able to clarify the whole functional connectome both in the patient and in the HC groups. They demonstrated many of the main differences, although full general and poorly detailed. Indeed, they weighed three series of network-to-network connections (intra-frontoparietal, intra-cerebellar, frontoparietal default) considered to exist of major importance for SCZ psychopathology and clinical manifestation (23). Another paper examined the role of long- and short range functional connectivity (lFC) (sFC) in discriminating patients from their own relatives or HC: SCZ group exhibited an spread in sFC and lFC in the DMN with an adequate level of accuracy, sensitivity, and specificity (94%, 92%, 96%, respectively) (27). By analyzing the coherence regional homogeneity (Cohe-ReHo) value, Liu et al. demonstrated that it was decreased in several areas, such as the left postcentral gyrus, correct precentral gyrus, left superior temporal gyrus, right middle frontal gyrus, left paracentral lobule, right IPL, and bilateral praecuneus in 48 SCZ vs 31 HC (26). The Whole encephalon ReHo measures were used as robust psychosis biomarker: SVM resulted more accurate in identify patterns of college ReHo abnormalities (inferior/heart temporal surface area and fusiform gyrus) (twoscore). The integration of the neuropsychological evaluation to detect dissimilar aspects related to attending, working memory, praxic, visuospatial, and executive functions was able for the early diagnosis of patients with SCZ (35).
The combination of SVM with other ML techniques can place anatomic brain areas with major alterations (temporal fusiform cortex, inferior, center, and medial frontal gyri, inferior temporal gyrus, anterior division of the parahippocampal gyrus, planum polare, cingulate gyrus, superior temporal gyrus, precuneus left, and right thalamus) with an accuracy close to 90% (21, 25). An farthermost learning machine (ELM) was developed past Qureshi et colleagues, reaching a maximum accuracy of 99.3%. Master data derived from cortical thickness and surface area, total cognitive volume, and overall volume of cortex features scans. Authors concluded that their ELM technique can be applied to patients offering a solid chance of helping clinicians to make diagnosis of SCZ (32).
Some other important field of application of SVM is the evaluation of functional features in first episode schizophrenia (FES). The identification of early-onset schizophrenia remains challenging, and SVM may constitute a promising tool for the early diagnosis for its high accuracy and valuable prognostic implication in FES. Recently, the sFC and lFC in the whole brain were explored in 48 first-episode, drug-naïve patients and 31 HC using SVM. Major abnormalities were found in some encephalon networks (anterior and posterior Default Way Network and Sensorimotor Network) classifying patients and controls with > 92% accuracy and high sensitivity and specificity (30). Liu et al. evaluated the alteration in FC in different brain regions in a similar patients' sample and found dysfunctional interhemispheric network within the sensorimotor surface area among patients with SCZ. Information technology was associated with processing speed deficits, indicating the probable interest with the neurocognitive alterations of these patients. The application of SVM ML technique analysis reached 100% sensitivity, 87.09% specificity, and 94.93% accuracy (34). Functional alterations could bespeak to a function of DMN and SN in the SCZ psychopathology that is already known in beginning-psychotic episode patients and SVM seems to be able to discriminate with high accuracy patients from HC in inquiry context. Wang et al. identify brain peculiarities using ReHo input in SVM analysis through resting state-fMRI (rs-fMRI) in drug-naïve patients and 32 HC. ReHo values were significantly amplified in the bilateral superior medial prefrontal cortex, and, otherwise, reduced in the left superior temporal gyrus, right precentral lobule, right inferior parietal lobule, and left paracentral lobule in patient group compared to HC (29). Disrupted functional asymmetry was calculated comparing patients with FES, drug-naïve schizophrenia, ultra-high run a risk (UHR) for psychosis and HC. SVM classification analysis was practical to analyze the data and showed decreased parameter of disproportion in the left thalamus/pallidum, right hippocampus/parahippocampus, right inferior frontal gyrus/insula, right thalamus, and left inferior parietal lobule, and increased PAS in the left calcarine, right superior occipital gyrus/eye occipital gyrus, and correct precentral gyrus/postcentral gyrus. First-episode patients and UHR subjects shared decreased pattern of functional asymmetry in the left thalamus underlining the possible involvement of the thalamus in the pathophysiology of psychosis and demonstrating a very early on marker for psychosis (41). A multimodal classification method to discriminate FES patients from HC combined structural MRI and rs-fMRI data, and identified functional markers in both gray matter and white affair and altered functional connectivity in DMN and cerebellar connections (36). A contempo study identified informative functional networks to distinguish patients from HC and to classify unaffected outset-degree relatives (FDRs) with or without functional networks similar to patients. Four informative functional networks (DMN, ventral frontotemporal network, and posterior DMN with parahippocampal gyrus) resulted implicated in encephalon alterations. They could be probably used as biomarkers to place FDRs with FN patterns similar to those of SCZ patients (38). The ability to apply complex mathematical calculations to big data is newly adult, and its use is hopefully growing. Now, theoretically, it is possible to create automatically models for analyzing larger and more than complex data and to produce more than authentic and repeatable results even on a large calibration.
The awarding of these models would allow clinicians to place new tasks, not merely diagnostic just also preventive, for major psychiatric disorders such equally Schizophrenia.
Decision
Approaches of big information, focusing on classification based on huge biological information rather than the single clinical manifestation, take the greatest advantage to move the field forward faster and with more evidence than before. The awarding of ML techniques in psychiatry as well, volition exist useful to routinely allocate patients with major psychiatric disorders, and schizophrenia in detail, on the ground of resting state functional MRI data. This technique can be a valid, inexpensive, and non-invasive back up for physicians to detect patients, even in the early on phase of the disorder, conferring a crucial diagnostic anticipation, hopefully decisive in changing the natural history of the affliction. The results collected in this review allow u.s. to assume that the greater accuracy demonstrated by the SVM models and new integrated methods of ML techniques could play an increasingly decisive function in the time to come both for the early diagnosis and a more accurate evaluation of the treatment response, and to establish the middle-term prognosis of patients with SCZ.
Author Contributions
All authors contributed to the article and approved the submitted version.
Funding
This research was co-funded by University "G. Fortunato" Benevento (Italian republic). Grant: cda N°viii/111119.
Conflict of Interest
The authors declare that the research was conducted in the absence of whatever commercial or financial relationships that could exist construed every bit a potential conflict of interest.
References
3. Simeone JC, Ward AJ, Rotella P, Collins J, Windisch R. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: A systematic literature review. BMC Psychiatry (2015) 15:193. doi: x.1186/s12888-015-0578-7
PubMed Abstract | CrossRef Full Text | Google Scholar
four. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-five. fifth Ed. Washington (2013).
Google Scholar
6. Kambeitz J, Kambeitz-Ilankovic L, Leucht S, Wood S, Davatzikos C, Malchow B, et al. Detecting neuroimaging biomarkers for schizophrenia: A meta-analysis of multivariate blueprint recognition studies. Neuropsychopharmacology (2015) forty:1742–51. doi: x.1038/npp.2015.22
PubMed Abstruse | CrossRef Full Text | Google Scholar
ix. He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, et al. Uncovering intrinsic modular system of spontaneous brain activity in humans. PloS 1 (2009) 4(4):e5226. doi: 10.1371/journal.pone.0005226
PubMed Abstruse | CrossRef Full Text | Google Scholar
ten. Han West, Sorg C, Zheng C, Yang Q, Zhang X, Ternblom A, et al. Depression-rank network signatures in the triple network separate schizophrenia and major depressive disorder. NeuroImage Clin (2019) 22:101725. doi: 10.1016/j.nicl.2019.101725
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Veronese E, Castellani U, Peruzzo D, Bellani M, Brambilla P. Machine learning approaches: from theory to application in schizophrenia. Comput Math Methods Med (2013) 2013:867924. doi: 10.1155/2013/867924
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Wolfers T, Buitelaar JK, Beckmann CF, Franke B, Marquand AF. From estimating activation locality to predicting disorder: A review of design recognition for neuroimaging-based psychiatric diagnostics. Neurosci Biobehav Rev (2015) 57:328–49. doi: ten.1016/j.neubiorev.2015.08.001
PubMed Abstruse | CrossRef Full Text | Google Scholar
13. Orrù G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using Support Vector Auto to place imaging biomarkers of neurological and psychiatric disease: A disquisitional review. Neurosci Biobehav Rev (2012) 36:1140–52. doi: 10.1016/j.neubiorev.2012.01.004
PubMed Abstract | CrossRef Full Text | Google Scholar
14. de Filippis R, Carbone EA, Gaetano R, Bruni A, Pugliese Five, Segura-Garcia C, et al. Machine learning techniques in a structural and functional MRI diagnostic approach in schizophrenia: a systematic review. Neuropsychiatr Dis Treat (2019) fifteen:1605–27. doi: 10.2147/NDT.S202418
PubMed Abstract | CrossRef Total Text | Google Scholar
15. Vapnik VN. An overview of statistical learning theory. IEEE Trans Neural Networks (1999) 10:988–99. doi: 10.1109/72.788640
CrossRef Full Text | Google Scholar
xvi. Krystal JH, Murray JD, Chekroud AM, Corlett PR, Yang G, Wang X-J, et al. Computational Psychiatry and the Claiming of Schizophrenia. Schizophr Bull (2017) 43:473–5. doi: x.1093/schbul/sbx025
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: caption and elaboration. BMJ (2009) 339:b2700. doi: ten.1136/bmj.b2700
PubMed Abstruse | CrossRef Full Text | Google Scholar
19. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-iv
PubMed Abstruse | CrossRef Full Text | Google Scholar
20. Su L, Wang L, Shen H, Feng G, Hu D. Discriminative analysis of not-linear brain connectivity in schizophrenia: An fMRI Study. Front Hum Neurosci (2013) 7:702. doi: 10.3389/fnhum.2013.00702
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Yang H, Liu J, Sui J, Pearlson G, Calhoun VD. A hybrid machine learning method for fusing fMRI and genetic data: Combining both improves classification of schizophrenia. Front Hum Neurosci (2010) 4:192. doi: 10.3389/fnhum.2010.00192
PubMed Abstruse | CrossRef Total Text | Google Scholar
22. Arbabshirani MR, Castro E, Calhoun VD. Accurate Classification of Schizophrenia Patients Based on Novel Resting-State fMRI Features. Conf Proc IEEE Eng Med Biol Soc (2014) 2014:6691–4. doi: 10.1109/EMBC.2014.6945163
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Watanabe T, Kessler D, Scott C, Angstadt K, Sripada C. Illness prediction based on functional connectomes using a scalable and spatially-informed back up vector machine. Neuroimage (2014) 96:183–202. doi: ten.1016/j.neuroimage.2014.03.067
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Koch SP, Hägele C, Haynes JD, Heinz A, Schlagenhauf F, Sterzer P. Diagnostic classification of schizophrenia patients on the basis of regional reward-related fMRI signal patterns. PloS One (2015) 10(three):e0119089. doi: 10.1371/journal.pone.0119089
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Chyzhyk D, Savio A, Graña Thou. Reckoner aided diagnosis of schizophrenia on resting country fMRI data by ensembles of ELM. Neural Networks (2015) 68:23–33. doi: 10.1016/j.neunet.2015.04.002
PubMed Abstruse | CrossRef Full Text | Google Scholar
26. Liu Y, Zhang Y, Lv L, Wu R, Zhao J, Guo W. Abnormal neural activity equally a potential biomarker for drug-naive starting time-episode adolescent-onset schizophrenia with coherence regional homogeneity and support vector auto analyses. Schizophr Res (2018) 192:408–xv. doi: 10.1016/j.schres.2017.04.028
PubMed Abstract | CrossRef Total Text | Google Scholar
27. Guo W, Liu F, Chen J, Wu R, Li L, Zhang Z, et al. Using curt-range and long-range functional connectivity to place schizophrenia with a family-based case-control blueprint. Psychiatry Res - Neuroimaging (2017) 264:60–seven. doi: 10.1016/j.pscychresns.2017.04.010
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Orban P, Dansereau C, Desbois 50, Mongeau-Pérusse V, Giguère CÉ, Nguyen H, et al. Multisite generalizability of schizophrenia diagnosis classification based on functional brain connectivity. Schizophr Res (2018) 192:167–71. doi: 10.1016/j.schres.2017.05.027
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Wang Due south, Zhang Y, Lv L, Wu R, Fan X, Zhao J, et al. Abnormal regional homogeneity as a potential imaging biomarker for adolescent-onset schizophrenia: A resting-state fMRI study and support vector auto analysis. Schizophr Res (2018) 192:179–84. doi: 10.1016/j.schres.2017.05.038
PubMed Abstract | CrossRef Full Text | Google Scholar
xxx. Wang Due south, Zhan Y, Zhang Y, Lyu L, Lyu H, Wang G, et al. Abnormal long- and curt-range functional connectivity in boyish-onset schizophrenia patients: A resting-land fMRI report. Prog Neuropsychopharmacol Biol Psychiatry (2018) 81:445–51. doi: x.1016/j.pnpbp.2017.08.012
PubMed Abstruse | CrossRef Full Text | Google Scholar
31. Bae Y, Kumarasamy G, Ali IM, Korfiatis P, Akkus Z, Erickson BJ. Differences Between Schizophrenic and Normal Subjects Using Network Backdrop from fMRI. J Digit Imaging (2018) 31:252–61. doi: ten.1007/s10278-017-0020-4
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Qureshi MNI, Oh J, Cho D, Jo HJ, Lee B. Multimodal discrimination of schizophrenia using hybrid weighted feature concatenation of encephalon functional connectivity and anatomical features with an extreme learning auto. Front Neuroinform (2017) 11:59. doi: ten.3389/fninf.2017.00059
PubMed Abstract | CrossRef Total Text | Google Scholar
33. Pläschke RN, Cieslik EC, Müller VI, Hoffstaedter F, Plachti A, Varikuti DP, et al. On the integrity of functional brain networks in schizophrenia, Parkinson'south disease, and advanced age: Bear witness from connectivity-based single-subject classification. Hum Brain Mapp (2017) 38:5845–58. doi: ten.1002/hbm.23763
PubMed Abstruse | CrossRef Full Text | Google Scholar
34. Liu Y, Guo W, Zhang Y, Lv 50, Hu F, Wu R, et al. Decreased Resting-Land Interhemispheric Functional Connectivity Correlated with Neurocognitive Deficits in Drug-Naive Outset-Episode Adolescent-Onset Schizophrenia. Int J Neuropsychopharmacol (2018) 21:33–41. doi: x.1093/ijnp/pyx095
PubMed Abstract | CrossRef Full Text | Google Scholar
35. Vacca A, Longo R, Mencar C. Identification and evaluation of cerebral deficits in schizophrenia using "Auto learning". Psychiatr Danub (2019) 31:261–4.
PubMed Abstract | Google Scholar
36. Zhuang H, Liu R, Wu C, Meng Z, Wang D, Liu D, et al. Multimodal nomenclature of drug-naïve outset-episode schizophrenia combining anatomical, diffusion and resting state functional resonance imaging. Neurosci Lett (2019) 705:87–93. doi: 10.1016/j.neulet.2019.04.039
PubMed Abstract | CrossRef Full Text | Google Scholar
37. Li J, Dominicus Y, Huang Y, Bezerianos A, Yu R. Machine learning technique reveals intrinsic characteristics of schizophrenia: an culling method. Brain Imaging Behav (2019) 13:1386–96. doi: 10.1007/s11682-018-9947-iv
PubMed Abstract | CrossRef Total Text | Google Scholar
38. Jing R, Li P, Ding Z, Lin X, Zhao R, Shi L, et al. Automobile learning identifies unaffected starting time-degree relatives with functional network patterns and cerebral impairment similar to those of schizophrenia patients. Hum Encephalon Mapp (2019) 40:3930–nine. doi: 10.1002/hb
PubMed Abstract | CrossRef Full Text | Google Scholar
twoscore. Ji L, Meda SA, Tamminga CA, Clementz BA, Keshavan MS, Sweeney JA, et al. Characterizing functional regional homogeneity (ReHo) as a B-SNIP psychosis biomarker using traditional and machine learning approaches. Schizophr Res (2019) 215:430–viii. doi: ten.1016/j.schres.2019.07.015
PubMed Abstract | CrossRef Total Text | Google Scholar
41. Zhu F, Liu Y, Liu F, Yang R, Li H, Chen J, et al. Functional asymmetry of thalamocortical networks in subjects at ultra-high risk for psychosis and first-episode schizophrenia. Eur Neuropsychopharmacol (2019) 29:519–28. doi: x.1016/j.euroneuro.2019.02.006
PubMed Abstract | CrossRef Total Text | Google Scholar
42. Zhou Y, Zeidman P, Wu S, Razi A, Chen C, Yang 50, et al. Contradistinct intrinsic and extrinsic connectivity in schizophrenia. NeuroImage Clin (2018) 17:704–16. doi: ten.1016/j.nicl.2017.12.006
PubMed Abstract | CrossRef Total Text | Google Scholar
43. Li P, Fan TT, Zhao RJ, Han Y, Shi 50, Lord's day HQ, et al. Altered Brain Network Connectivity every bit a Potential Endophenotype of Schizophrenia. Sci Rep (2017) 7(1):5483. doi: 10.1038/s41598-017-05774-iii
PubMed Abstract | CrossRef Full Text | Google Scholar
44. Bisenius Southward, Mueller K, Diehl-Schmid J, Fassbender Grand, Grimmer T, Jessen F, et al. Predicting chief progressive aphasias with support vector auto approaches in structural MRI data. NeuroImage Clin (2017) 14:334–43. doi: x.1016/j.nicl.2017.02.003
PubMed Abstract | CrossRef Full Text | Google Scholar
45. Pellegrini Eastward, Ballerini L, Hernandez M del CV, Chappell FM, González-Castro V, Anblagan D, et al. Auto learning of neuroimaging for assisted diagnosis of cerebral harm and dementia: A systematic review. Alzheimer's Dement Diagnosis Assess Dis Monit (2018) 10:519–35. doi: x.1016/j.dadm.2018.07.004
CrossRef Total Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00588/full